How to Produce Ice Crystals in Clouds
- By Vincent Schaefer
- Feb 19, 2021
This paper from 1948 describes the laboratory experiment that Vincent Schaefer created for seeding a supercooled cloud and converting it to ice crystals. This was part of his work at the GE Research Laboratory in Schenectady, New York and provided the foundation for weather modification and cloud seeding experiments.
Please note that this is not a recipe for science experimentation at home. National Geographic and other sites do, however, have detailed activity instructions for making your own snow and ice crystals at home.
The basic experiment in which a supercooled cloud of liquid water droplets may be converted to a mass of floating ice crystals is a very simple one. It may be worthwhile at this time to describe the method in greater detail so that others may use it for demonstration purposes or as a starting point for further research.
The essential items for the experiment in its simplest form are:
- An open top cold chamber having a volume of one to four cubic feet which may be cooled to a temperature of about -10°C
- A light source which will give a strong concentrated beam, and
- A fragment of dry ice
The first two items may consist of simple homemade units or they may be assembled from standard pieces of equipment.
The Cold Chamber
In its most convenient form, which is most suitable for classroom or laboratory demonstrations and use, the cold chamber is a standard home freezer unit of four or eight cubic foot capacity. Such units are made with a lift top and will, when set for maximum cooling, show an average air temperature in the center of the chamber of about -15 °C with the top kept open. A simpler unit of much lower cost consists of two square containers, such as galvanized wash tubs, which when nested are separated by a space of at least three inches on the bottom and sides with the top rims being level with each other.
A cooling solution of snow or chipped ice and sodium chloride in the ratio of three parts by weight of snow or ice to one part of salt will cool the inner chamber for at least a day if the outer container is protected with at least three inches of thermal insulation.
Whether a standard home-freezer unit or a homemade cold chamber is used for the experiment, a temperature gradient occurs in which it is coldest at the bottom. This inversion, which often exceeds 10C° per foot, greatly stabilizes the air.
In order to observe the beautiful effects that occur when the transformation experiment is made, the cold chamber should be lined with black cloth, such as velvet, or coated with a flat black paint.
The Light Source
Various types of illuminators may be used effectively. The writer has used everything from a high pressure water-cooled 1000 watt mercury capillary lamp (Type AH6) to a small flashlight. The water-cooled lamp is necessary if colored photographs or movies are made, but a 150 watt projection spot lamp or a 100 watt air-cooled mercury capillary lamp (Type AH4) fitted with a cylindrical lens provides excellent illumination for ordinary demonstration and study purposes and black and white photography. Other suitable illuminators are flashlights using 6- or 12-volt batteries or 6-volt automobile spotlights. Ordinary 2-cell flashlights do not provide sufficient light for good optical effects.
The Formation of a Supercooled Cloud
The first part of the experiment involves the production of a supercooled cloud. This is easily accomplished by introducing a single breath into the cold chamber by exhaling with the mouth placed close to the top rim of the chamber. The moist air from the breath immediately condenses to form a cloud. Under ordinary conditions, this cloud consists of liquid water droplets from 10-15 microns diameter with a liquid water content ranging between 0.5 and 1 gram per cubic meter. Such clouds can also be made by waving a damp cloth through the cold air or by placing a saturated porous porcelain container on the floor of the chamber until a dense enough cloud forms. When such a cloud is formed in a chamber having a temperature of -15 °C, it is a common occurrence to have the cloud develop without a single ice crystal present. With the illumination and dark background recommended in the earlier part of this paper, it is easy to see a single crystal in the cloud of water droplets. The supercooled cloud, if left alone after being formed, will slowly disappear mostly by evaporation onto the frosted walls and floor of the chamber.
A simple experiment can be performed to prove that the cloud is supercooled. If a wire 0.005" in diameter and 4" long is put on the end of a shaft extending from a small electric motor and caused to rotate at 1800 RPM (a small stirring motor is ideal for the experiment), a coating of rime ice will form on the wire within a few seconds. As soon as this is removed from the chamber, the ice melts and forms tiny water droplets along the wire with the larger ones nearer the outer end of the wire where the speed is greatest.
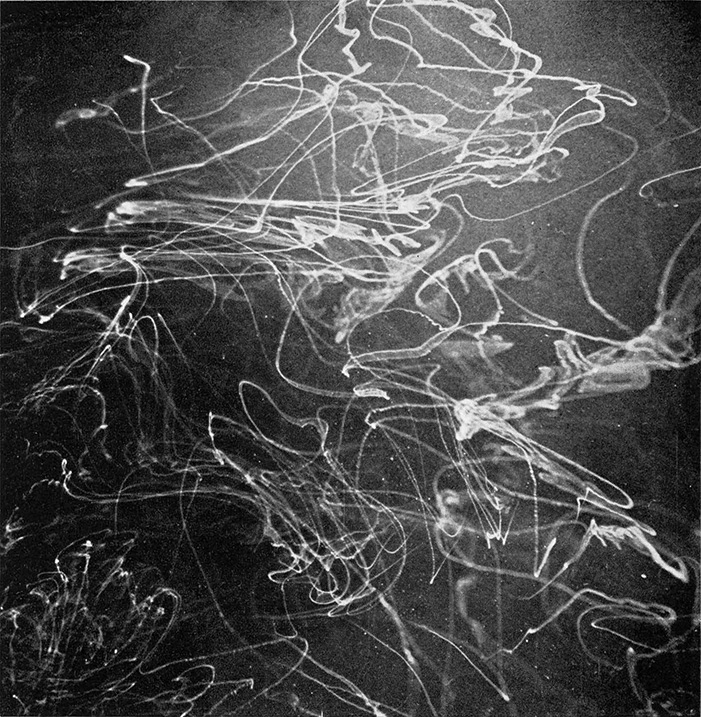
Figure 1a. Typical trails of minute ice crystals produced by tiny fragments of dry ice falling through a supercooled cloud.
The Seeding Material
The supercooled cloud may be converted to ice crystals in two ways, either by chilling a small region of the cloud below -39 °C or by introducing foreign particles of a specific crystalline form into the supercooled cloud. The formation of submicroscopic ice crystals by cooling a localized region of the cloud below the transition temperature is most easily accomplished with solid carbon dioxide (dry ice).
Dry ice is most convenient in its compressed form, such as used for shipping frozen foods and ice cream. It may be obtained from most dairy establishments. The block should be handled with gloves or some other protective insulation. Dry ice can be produced by inverting a tank of liquid CO2 and collecting in a cloth bag the snow-like residue which forms when the valve is opened. This may then be compressed into the form of a block or pellet. A very convenient dry ice generator is commercially available which permits the formation of about a gram of solid CO2 from tiny CO2 containers.
THE FORMATION OF ICE CRYSTALS
Various techniques may be employed to show the seeding process in which the supercooled cloud is changed to one containing only ice crystals. One of the most beautiful effects follows when a piece of dry ice is held in the warm air above the chamber containing the supercooled cloud and scratched with a nail. Tiny fragments of dry ice fall through the air, enter the cloud, and leave beautiful white trails as they fall to the bottom of the chamber. These look like vapor trails left in the sky by a plane. Figures 1a and b show a typical effect observable.

Figure 1b. Ice crystals in chamber less than a minute after initial seeding operation with dry ice
These tiny white trails immediately start growing in cross section as slight convection currents develop and little rings of chilled air sink below the initial seeded trail. Within a few seconds all along these regions twinkling crystals may be seen. Laboratory experiments indicate that the initial growth of these crystals proceeds with tremendous speed, increasing at least a billion fold in volume within a few seconds. The experiments also show the tremendous quantities of crystals which are generated from a tiny fragment of dry ice. At least 1016 primary nuclei may form from a pellet of dry ice about 1 cm in diameter. If all of these grew to the size of a small snow crystal, 300,000 tons of snow would result.
A very interesting and important physical phenomenon may be seen if turbulence in the supercooled cloud is allowed to die out before the cloud is seeded. After the cloud becomes stratified (within about one minute), the dry ice fragments should be introduced with a slow motion. This effect appears as a dark sheath free of both ice crystals and supercooled water droplets. It separates the region containing ice crystals from the supercooled cloud and is the diffusion path which develops as the liquid-water droplets evaporate, the vapor passing irreversibly to the ice crystals. This occurs since liquid water has a higher vapor pressure than ice at all temperatures below 0°C. Under favorable conditions, this vapor sheath often grows to at least 1½ cm in width before it disappears due to local turbulence.
The effect of the difference in vapor pressure between water and ice at temperatures below 0°C is beautifully illustrated if, after seeding the supercooled cloud, an attempt is made to form a new one. If the temperature of the air in the cold chamber is about -10 °C and a dozen or so tiny grains of dry ice are permitted to fall into the cloud, any tendency to form a supercooled cloud will be impossible until the newly formed crystals grow large enough to fall to the floor of the chamber. This will occur within four or five minutes if they are supplied with a continuing supply of moist air.
The falling velocity of crystals measuring about 50 microns (0.002") across the flats of the hexagon is great enough to make it difficult to keep them suspended in the air of the chamber. Depending on the type of crystal produced, this falling velocity may exceed 1 cm per second, the hexagonal columns and asymmetric forms always falling faster than the plates.
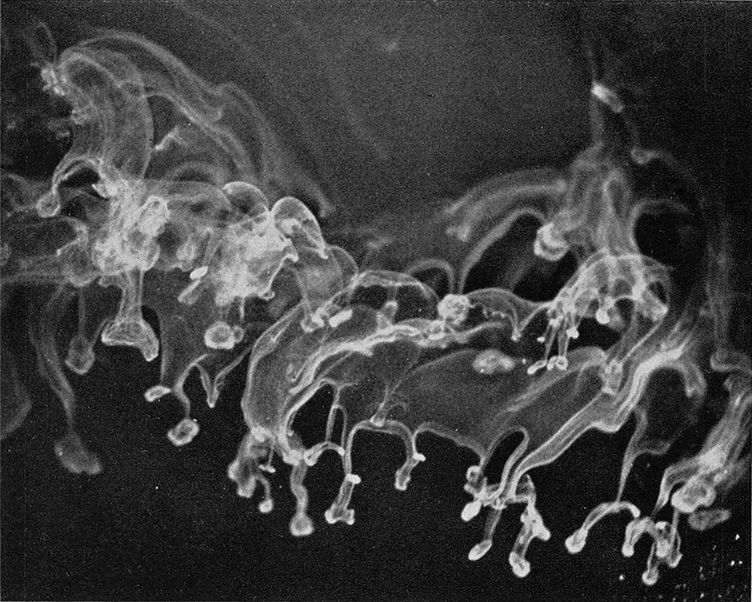
Figure 2a. Trail of ice crystals produced in supercooled cloud by passing a sewing needle cooled below -40 °C
That dry ice is effective only because of its cold temperature has already been shown. In the initial experiments, however, it was not possible to get an accurate measurement of the temperature of the transition point. Since that time a more exact method has been devised using a closed copper chamber fitted with thermocouples and accurate thermometers.
After a large number of experiments, it was found that the transition temperature seems to be -39 °C, +0.1. A few crystals were always observed at temperatures as high as -35°C although they were always seen floating in a supercooled cloud and are believed to be caused by foreign particles serving as sublimation nuclei.
It was found that if a drop of mercury (M.P. -38.89°C) was made solid with liquid air and passed slowly through a supercooled cloud, it would leave ice crystals in its wake so long as it remained solid. At the instant that it melted, however, this property disappeared.
FIGURES 2a and b illustrate two steps which are phases in the growth of ice crystals when a needle cooled with dry ice or liquid air below -40°C is passed slowly through a supercooled cloud having a temperature of -5°C
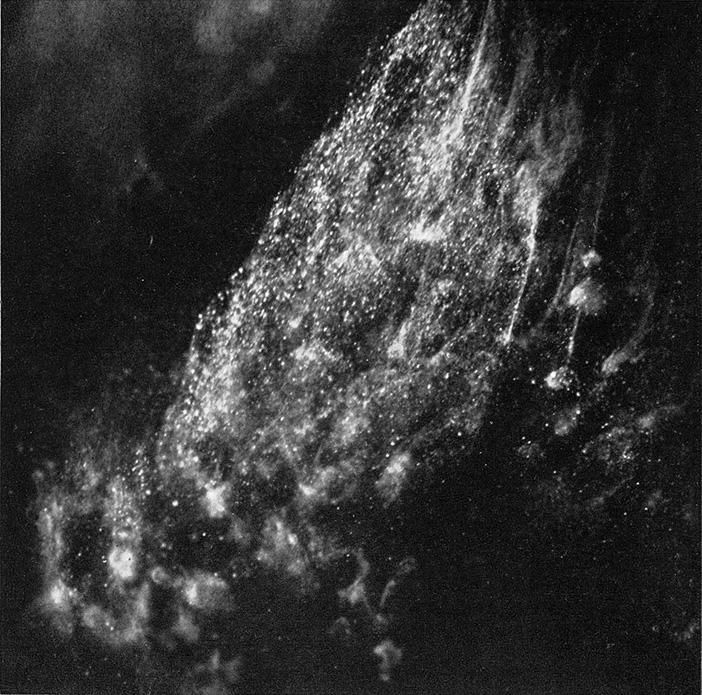
Figure 2b. Ice crystals in chamber within thirty seconds. Upper left shows part of unseeded supercooled cloud
SNOW CRYSTAL TYPES FORMED IN COLD CHAMBER
After growing the crystals in the chamber to the size where they start to “snow out,” it is a simple matter to examine them under the microscope. While this may be done by placing a microscope into the chamber, a more satisfactory method for the study of such crystals is the preparation of plastic replicas of them. This is a simple process:
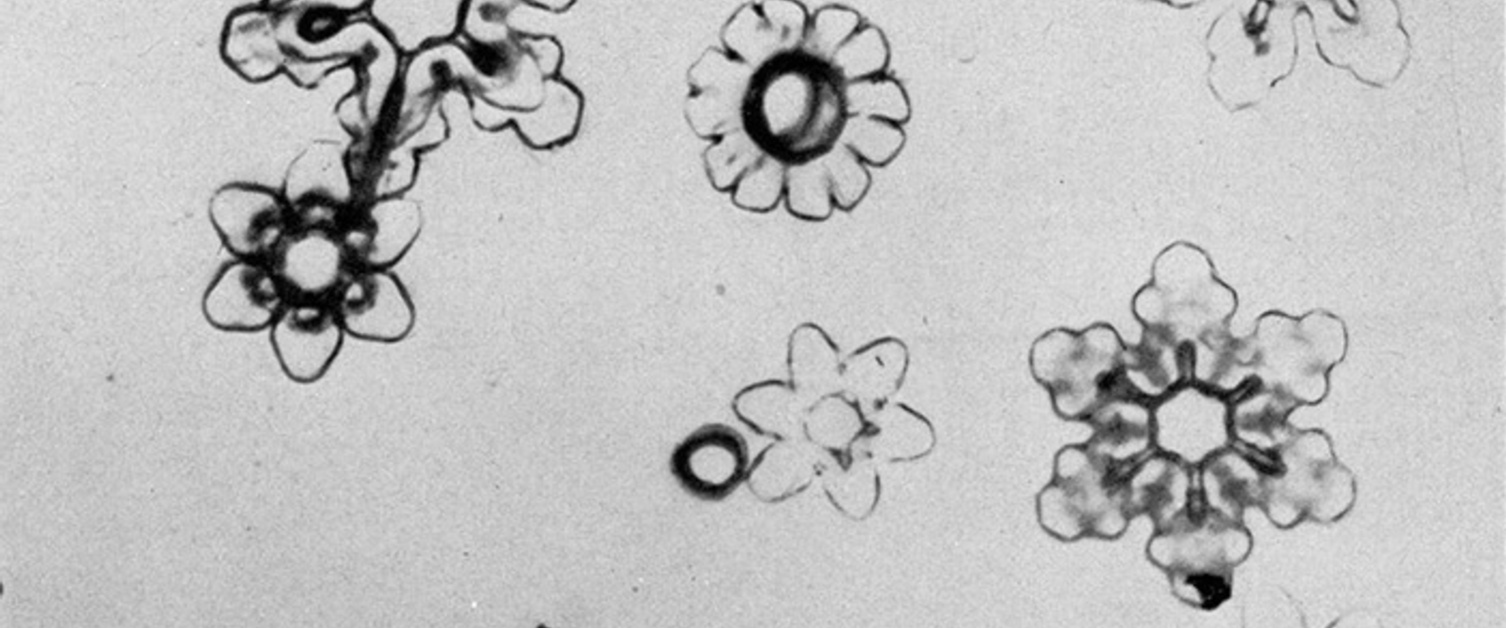
A one per cent solution of polyvinyl formal dissolved in ethylene dichloride is placed in the chamber and allowed to cool to a temperature of -5°C. Whenever it is desired to obtain a representative sample of the ice crystals in the chamber, a clean microscope slide is lowered into the solution, held in it for about 30 seconds, withdrawn and placed in a horizontal position for a period ranging from ten seconds to two minutes depending on the concentration of crystals in the chamber, their size, and the density wanted on the sampling slide. After the desired exposure has been made, the slide should be turned over and kept in the same region of the cold chamber. Within a few minutes, the solvent will evaporate leaving the crystals encased in thin, but hard and tough, plastic shells. This coating retains in microscopic detail all of the surface configurations of the original crystals. Once the solvent has all evaporated and the surface appears dry, the slide may be removed from its cold environment and warmed to room temperature. As this occurs the crystals melt, the water residue passing through the thin coating as it evaporates. FIGURES 3a, b, and c shows typical photomicrographs of replicas obtained in the cold chamber when seeded with dry ice. These are identical in crystal form to those which form at cirrus levels in the natural atmosphere and in temperature inversions close to the earth on cold clear mornings.
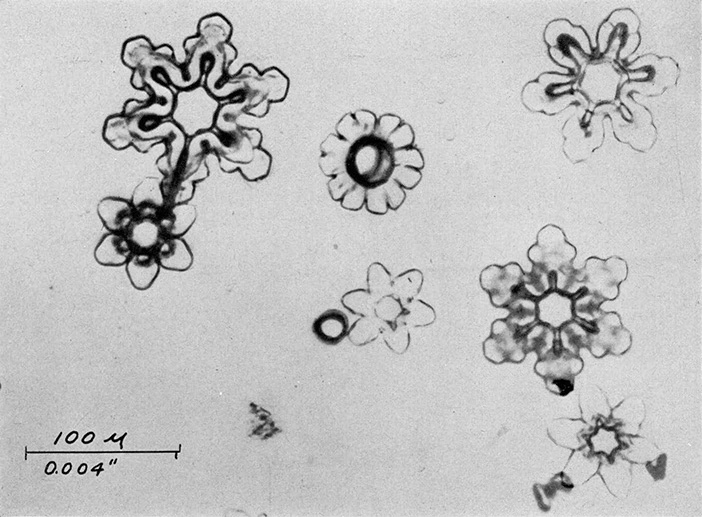
Figure 3a. Typical stellar crystal forms produced in cold chamber
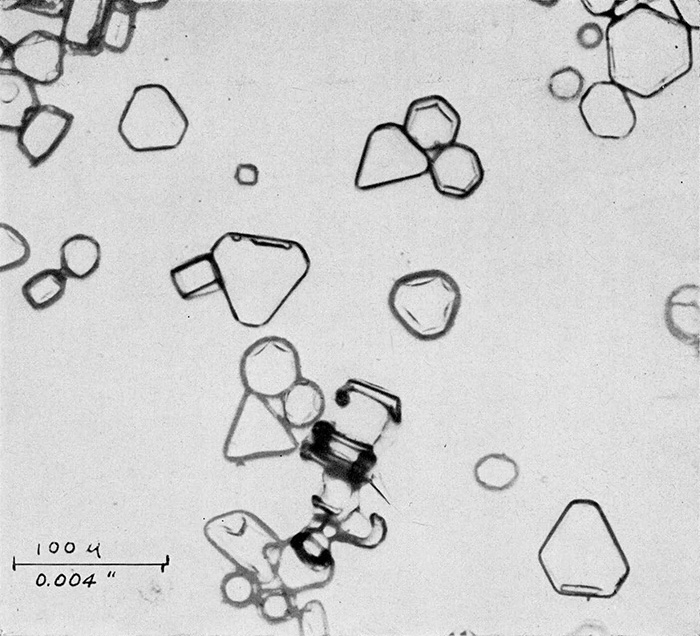
Figure 3b. Types of trigonal and hexagonal ice crystals formed in cold chamber
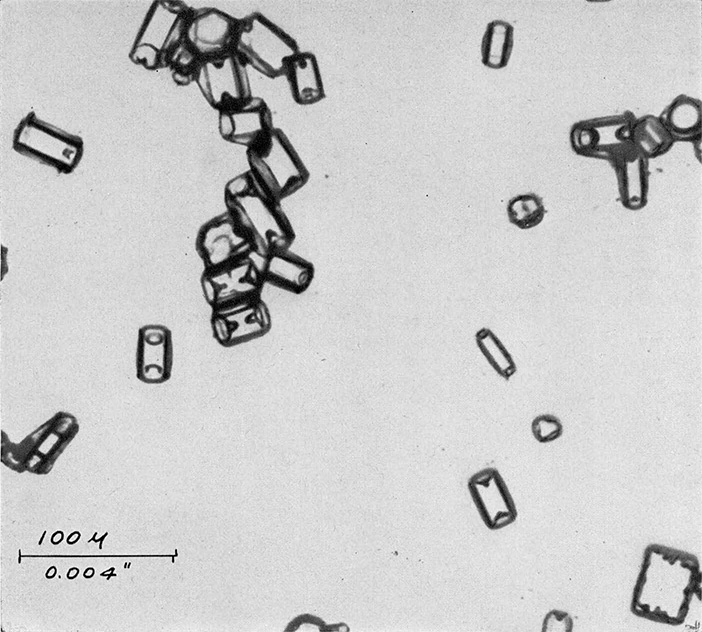
Figure 3c. One of the varieties of hexagonal columns formed in cold chamber
More information on Vincent Schaefer's work at GE and the weather modification experiments can be found here.