Snowflakes are Six-Sided (not Four- or Eight-sided), and other Ice Crystal Shape Considerations
- By Lourdes B. Avilés, Ph.D.
- Dec 8, 2023
Lourdes B. Avilés, Ph.D.
For many years I have been paying attention (one might say, too much attention) to the number of sides on any snowflake representation I encounter in decorations, jewelry, art, or even food items. I love finding six-sided snowflakes, but the ones that are four- or eight-sided make me cringe a little bit. Once at a craft show, a vendor was trying to convince me to buy a beautiful necklace that had caught my attention, until I noticed that the snowflake pendant had eight sides, and after a small dose of high-pressure salesmanship I had to tell them why I could never change my mind about buying it. I normally don’t say anything, although I might tell an accompanying friend that scientifically accurate snowflakes are six-sided and most definitely cannot be eight-sided.
I can sometimes be loud about it. Years ago, for example, when I was teaching an evening severe weather course for non-meteorology students, I told them of my horror at seeing four-sided paper snowflakes at the local grocery store the previous evening. One of the students immediately let me know that it was his wife who made them. Oops. After considering for a second, and knowing I had a good rapport with the students, I told him something like “you tell her that her snowflakes are WRONG” (I might have also done some kind of pirouette at the same time), and we all laughed about it.
My obsession might seem a little extreme, but please know that it is not about judging the scientific infraction, as much as an immediate feeling of responsibility to educate the perpetrator out of their errant ways. Ok, that DID take an extreme turn, but I would like to think that this crusade of mine is harmless. It will obviously never lead to saving the world or anything like that; but regardless, I valiantly continue my self-appointed mission to rid the world of incorrectly sided snowflakes. I must confess, however, that I once bought a bag of FIVE-sided yogurt pretzels labeled as “snowflakes” (I guess they were stars instead?), going against my never-buy-incorrectly-sided-snowflakes rule, while rationalizing that by eating them I would be removing them from the world.
Funnily, over the years, I seem to have successfully gotten to students, colleagues, and friends, some of whom have reported that they make sure their snowflakes are six-sided before there is any chance that I will see them.

Even though it is easier to make four- or eight-sided paper snowflakes, they do not represent scientifically accurate earthly ice crystals. Fortunately, it is not too hard to instead make them six-sided. Instructions are shown below.
Much can be written about snowflakes, or more explicitly, about ice crystals that can grow naturally or in an appropriately equipped lab into what we call snowflakes. Entire books have been written from the perspective of different disciplines and aimed at a variety of both technical and amateur audiences, and not surprisingly, at children. Visually, they certainly capture our imagination. Photographs of both natural and artificially grown snowflakes can be hauntingly beautiful. Many of these pictures are testaments to sophisticated techniques and technology, magnificent pieces of art taken by scientists or professional photographers. These days, however, even regular people with cell phones like you and I can photograph them well-enough to feel proudly excited.

Snowflake photos taken with a cell phone camera. “I keep my striped sweater and sometimes purple glove in the unheated garage to catch flakes. I have a stick-on magnifying lens I can put on my phone's lens. It's best if there isn't a lot of snow coming down because it's annoying to find a nice flake and then others land on it.” Courtesy of Beth Katz – taken in Lancaster County, Pennsylvania
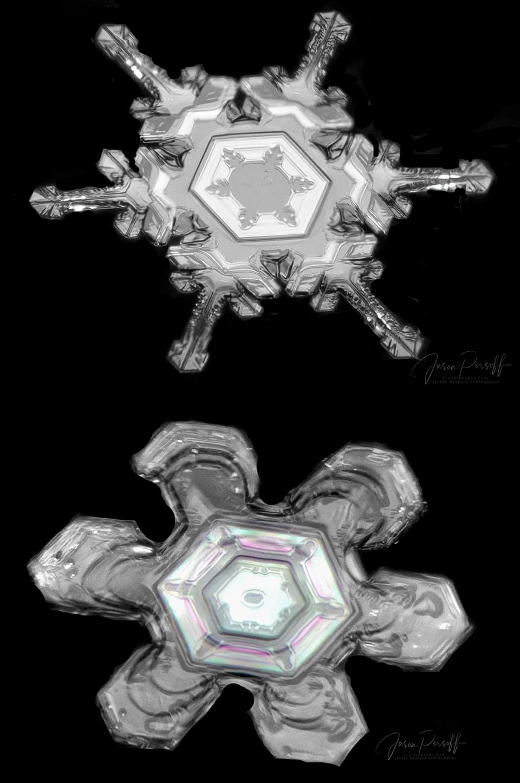
Snowflake photos taken with a DSLR camera and macro lens. Courtesy of Jason Persoff, who explains his technique in detail in a How to Photograph and Process Snowflake Photos - YouTube Playlist

Photo by Kenneth Libbrecht, professor at the California Institute of Technology (Caltech) who has been studying the physics of snowflakes and photographing them for decades. Used with permission. See SnowCrystals.com for more about the science of snowflakes, many more pictures, and information about Dr. Libbrecht’s books.
Ice crystals are solid water formed by water vapor molecules attaching themselves onto other water molecules in specific lattice arrangements that are energetically and structurally favorable at temperatures below freezing (a little more about this below). On Planet Earth and its atmosphere, ice crystals can take on many different shapes, but all of them have some kind of hexagonal symmetry; in other words, our ice crystals are always six-sided in one way or another. The ones that come to mind when we think of snowflakes are called dendrites and they are extreme versions of basic hexagonal plates (flat hexagons) that have grown protrusions and intricate arms. This can happen most easily when the temperature inside the cloud where the crystal is growing is within a very specific range of (from a couple of degrees colder than –14°C to a couple of degrees warmer) and at the same time the amount of moisture available for it to grow is very high (meaning that there are so many water vapor molecules available in the air that enough of them can easily attach to the growing ice crystal arrangement).
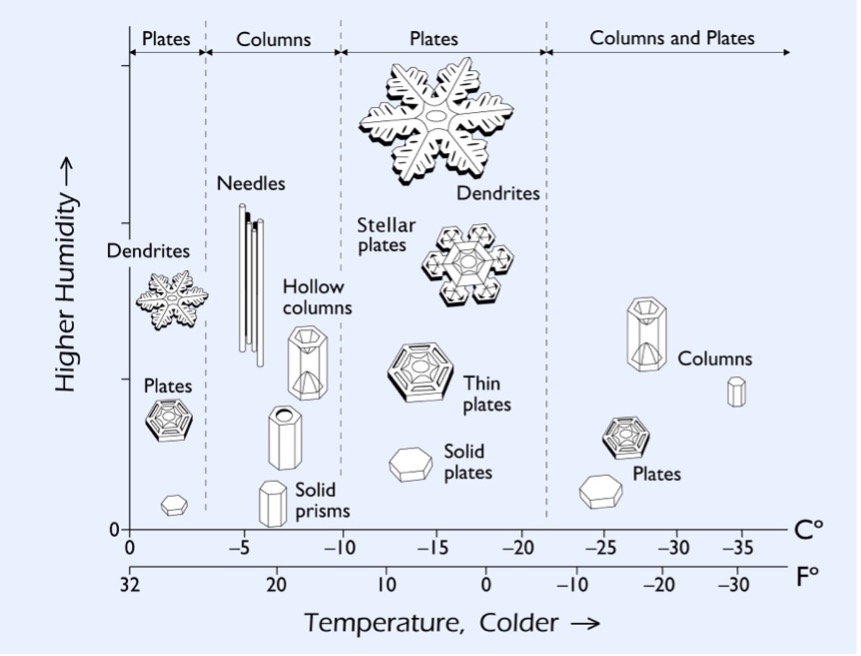
The type of ice crystal that can form and its most intricate shape possible at higher levels of humidity vary for different cloud temperatures. As a growing ice crystal moves inside a cloud, it is exposed to a variety of temperature/humidity conditions and shifts growth modes accordingly. As two crystals are unlikely to follow exactly the same conditions, this gives credibility to the saying of “no two snowflakes are ever alike,” at least to a first approximation. A cloud temperature of –14°C provides the most favorable conditions for water vapor molecules to deposit onto a growing dendrite, which in turn can produce the greatest snowfall accumulations. Because of this, a forecaster trying to determine snowfall accumulations for a certain amount of water available for precipitation will sometimes refer to a cloud layer with temperatures roughly —13°C to –15°C as the dendritic growth zone. (Diagram courtesy of SnowCrystals.com. Used with permission.)
There is much more that we would cover in say, an atmospheric physics course. For example, we could talk about the needed levels of supersaturation (excess water vapor in the air), or we could go deep into physical chemistry considerations (water is weird, by the way… see more about that below), or even cover topics like crystallographic symmetry and rotational modes. We could also discuss how turbulence affects how the different types of crystals fall through the atmosphere and how that might cause or inhibit their attachment to other crystals (what is called accretion) or cause water droplets freezing onto them (called rhyming), or how snowfall accumulations on the ground are higher when large dendrites fall from the clouds than when simpler crystals fall. We will not go far or at all toward those directions here. It is interesting, however, to acknowledge that there are so many possibilities to explore. We will mostly focus on the shape of ice crystals.
Ice crystal “habits”
In an introductory meteorology course, one might learn about four basic ice crystal habits (terminology sometimes used to refer to the external shape of an individual crystal): columns, needles, plates, and dendrites. Or maybe in an upper-level course, one might hear that they are all columns and plates with needles and dendrites being their extreme versions (for favorable temperature ranges and high levels of supersaturation). Alternatively, one might learn how it is all one basic shape, a hexagonal prism that can range from a thin long column to a flat wide plate and everything in between, and with a large variety of accessories possible. One might also explore one or more of the various ice crystal classification systems, which feature specialized shapes, as for example, sectored plates, capped columns, bullet rosettes, and many other tantalizingly named crystal possibilities. The simplest, cleanest forms (smooth, solid plates and columns) are the ones that can give us beautiful and exciting atmospheric optics: ice halos of all types that form via the refraction and reflection of sunlight through and off such crystals. That is a story for another day, however. Our crystals of interest, the dendrites, are too complicated to produce optical effects.

Types of ice crystals one might find in nature. The bottom rightmost cases are not formed purely by deposition: a rimed ice crystal has frozen droplets attached, while graupel forms when riming continues to the point where the original crystal shape is lost. (Diagram courtesy of SnowCrystals.com. Used with permission.)
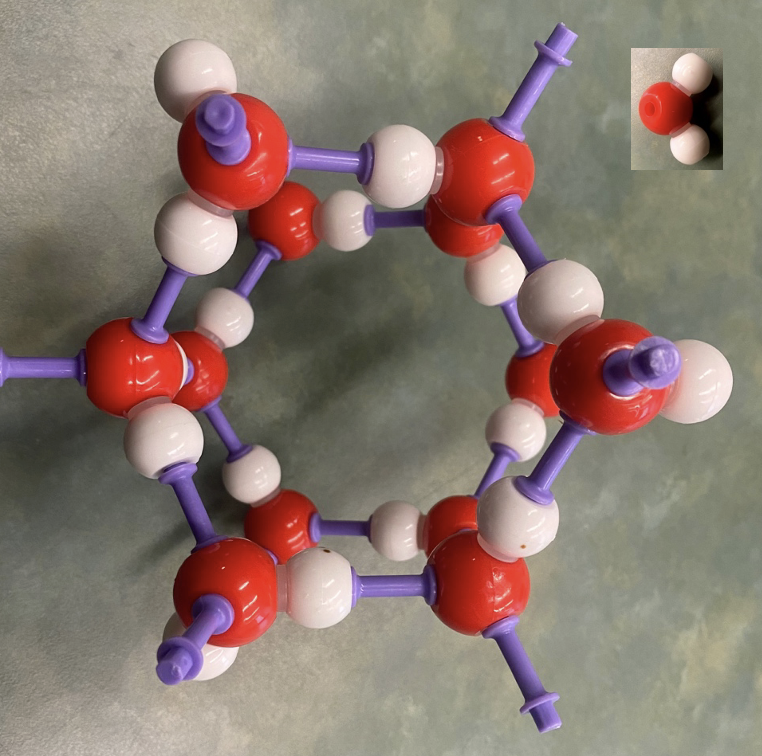
A simple molecule chemistry set represents the hexagonal structure of ice crystals. The insert shows a representation of the water molecule, H2O. The purple connectors represent the connections between each oxygen atom and two hydrogen atoms. A three-dimensional representation is needed to better see how the six-sided structure develops.
Water is weird… hexagonal ice, revisited
Through the process of deposition, water vapor molecules in the air join a group of molecules already arranged in a solid lattice, in other words, water goes straight from its gas form to its solid form. This is very different than an ice cube in your glass of water, which formed by freezing water from liquid to solid inside your freezer. It is also different from sleet or from any other type of frozen or freezing precipitation that entails liquid water freezing (including freezing rain, graupel, and hail). One molecule at a time, ice crystals grow into one of the possible simple or intricate six-sided shapes as they are carried around inside clouds. Sometimes, tiny droplets with temperatures below freezing, freeze on contact with the growing ice crystals. A lot of interesting things can in fact happen when so-called supercooled water (temperature below freezing but still in liquid form) freezes onto things, but that is also a story for another day.
It turns out that water is a very strange substance. We never think about it, since it is so familiar and important to life on Earth, but you might remember for example, from middle school science that water is the only common liquid that becomes less dense when it freezes—which is why ice cubes float. From a deeper recollection, you might also remember from high school chemistry those diagrams that arrange gas (higher temperatures and/or lower pressures), solid (lower temperatures and/or higher pressures), and liquid (in between temperatures/pressures—and very substance dependent), separated by lines that mark the boundaries between the three common phases of matter. You can draw them for any substance, the region of the phase diagram corresponding to water is a lot more complex than one might expect. For different temperature and pressure ranges, we have ice with different characteristics and behaviors, almost twenty different types! The one that we know and love on Planet Earth is Ice Ih (hexagonal ice, one-h), since the ranges of temperature and pressure that are possible throughout the Earth System neatly fit within the conditions for which this type can form.
Guess what can happen at really, really, cold temperatures: CUBIC ICE! This is ice Ic (one-c), a similar type of ice as our six-sided friend, but with four-sided symmetry! Physical chemists and crystallographers of various inclinations are very much aware of this, but it was a bit of a revelation for me as I learned about the different types of ice. Therefore, four-sided (hence also eight-sided) ice crystals ARE possible, BUT not at the temperatures and pressures where clouds form and snowflakes grow and fall to the ground. The average temperature in the coldest part of the atmosphere (in the mesopause, where the mesosphere ends and the thermosphere starts, way above the troposphere where clouds and precipitation are), is approximately minus one hundred degrees Celsius (–148°F). At these temperatures, it is possible for cubic ice to form (and it can survive to higher temperatures once formed). Still, ice crystals at those heights, regardless of their shape, would not have a chance to grow into snowflakes as we know them. Therefore, it seems that my efforts to rid the world of four- and eight-sided snowflake decorations are still scientifically justified!
Paper snowflakes
Back in 2010, after a few years of teaching that severe and hazardous weather course, it finally occurred to me that a fun activity I could do with students was to learn how to make six-sided paper snowflakes (thus growing my own army of six-sided paper snowflake makers that could go into the world and spread the word). A few years later, I decided to start doing it with upper-level meteorology majors and graduate students in atmospheric physics. I am happy to report that both groups of students enjoyed the activity, and I have loved seeing their many creations over the years.

A collection of paper snowflakes made by students in my atmospheric physics course as a fun exercise to complement the discussion and accompanying mathematical derivations related to microphysical cold cloud precipitation processes.
The basics of paper snowflake-making consist of a handful of simple folding and cutting tricks. I made the visuals below for my students in the severe weather course mentioned above. You can follow the pictured steps to make snowflakes to your heart’s contentment. When unfolding your creation, a beautiful six-sided snowflake will reveal itself.... in theory… as you can also disappointingly end up with six separate pieces of paper if you accidentally cut off the entire fold that keeps the structure together. If you do not cut enough negative space off, you might also have something that resembles a mostly circular crocheted doily. If either of those happen, do not give up. Even those of my students that declared that they would never be able to do it, did do it.
Here are the steps:
1. Cut a blank piece of paper such that you have a perfect square. An easy way to do this is to fold one corner until you reach the edge of the paper and then cut off the excess.
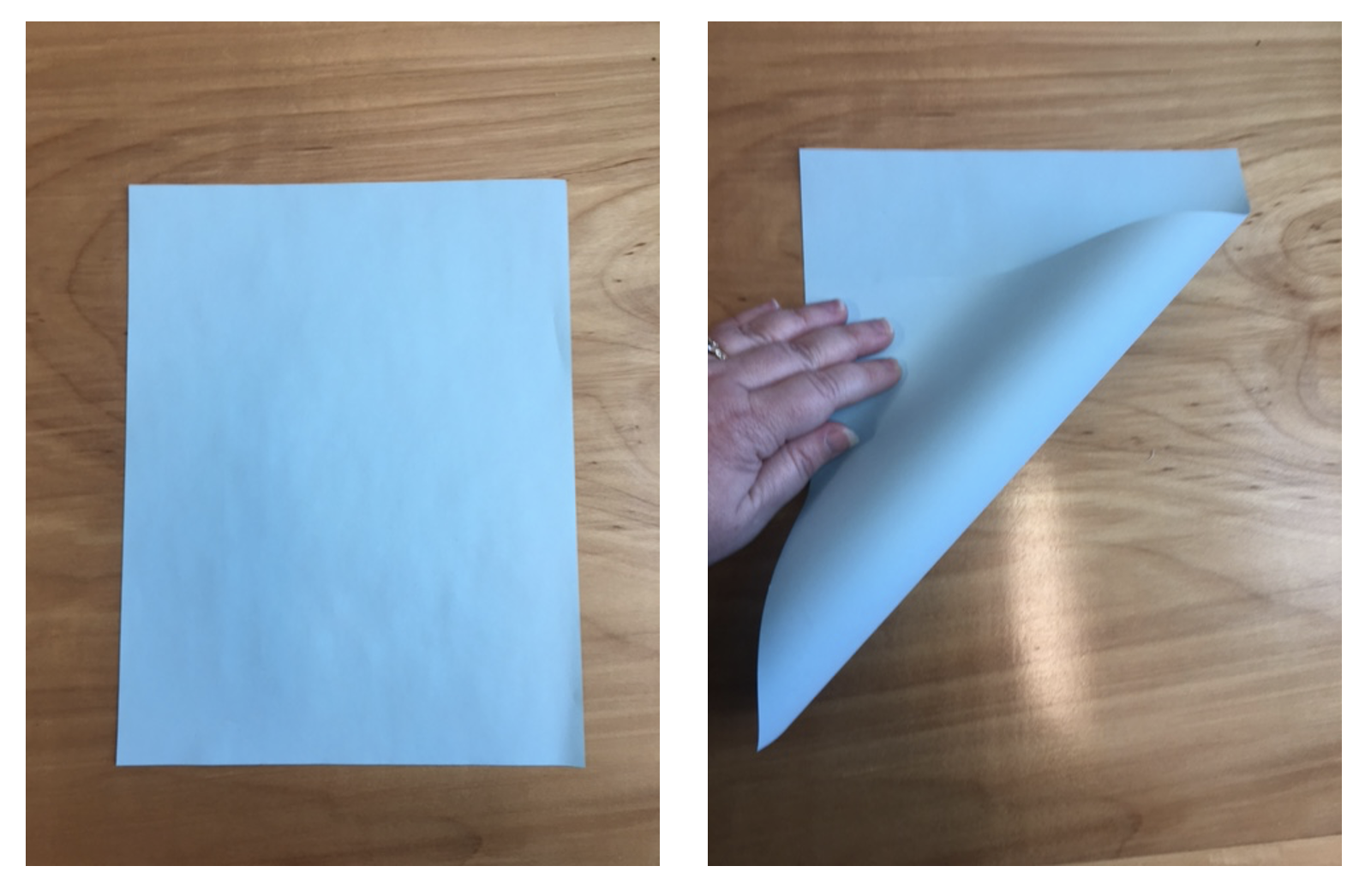
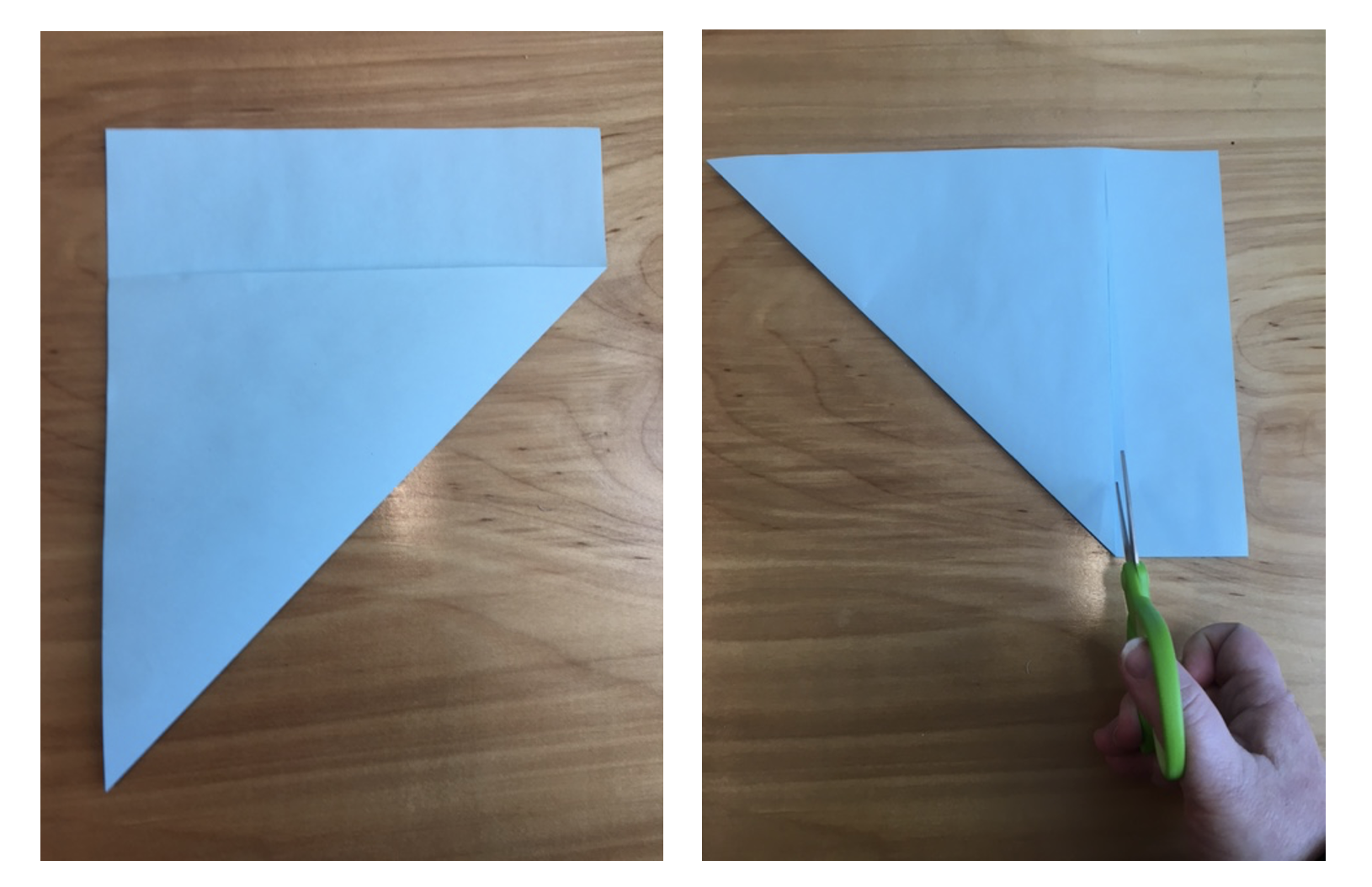
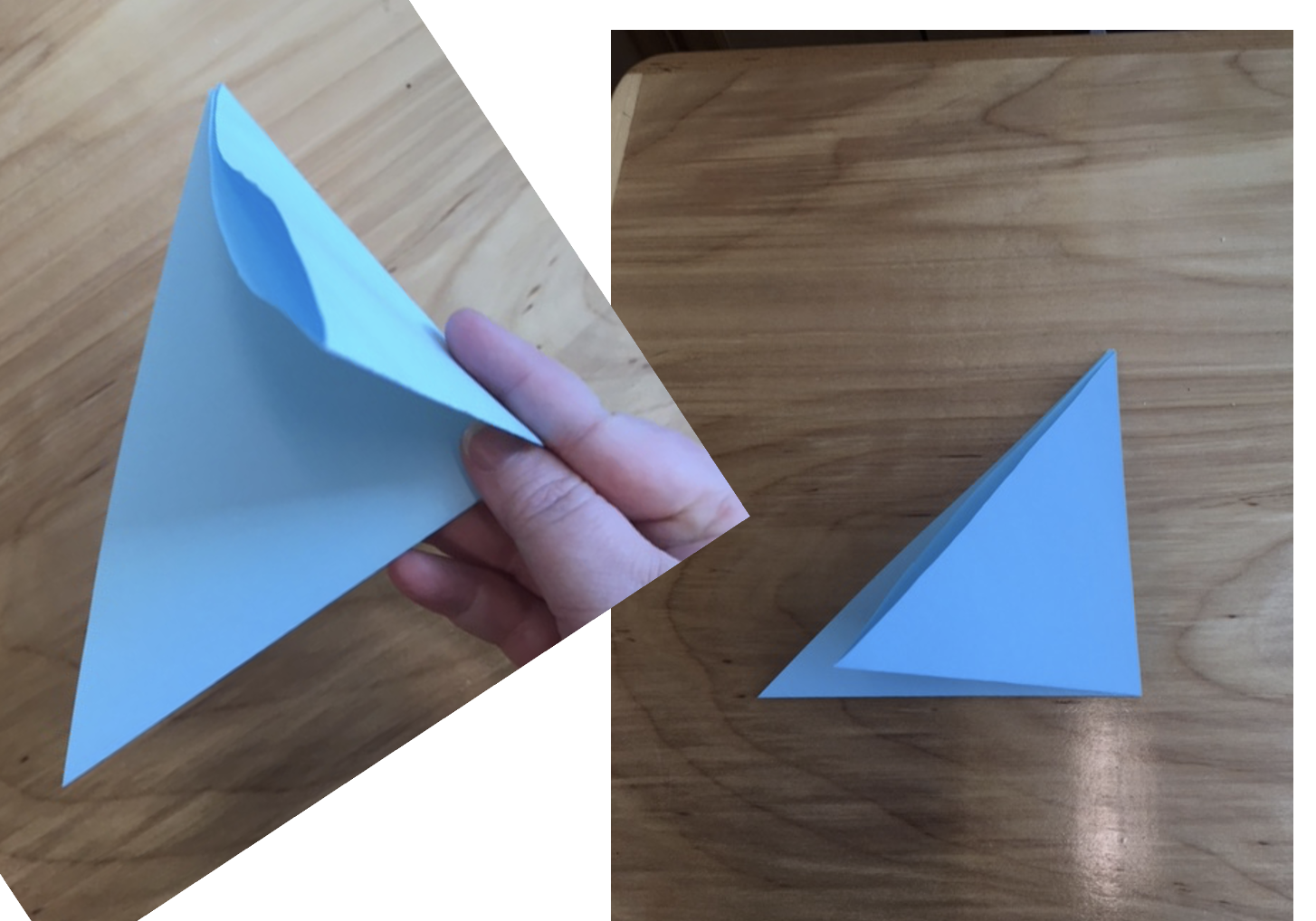
2. The folded square is a triangle. Fold that triangle again into a smaller one.
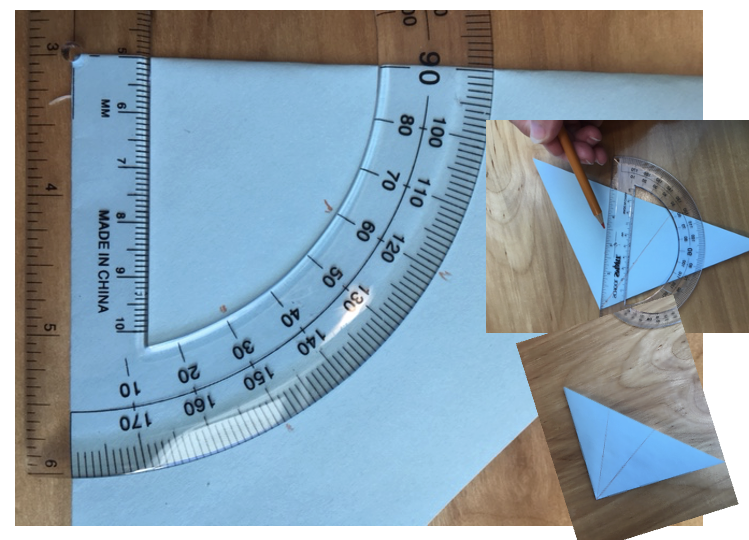
3. Fold the small triangle such that you have three sections (a ninety-degree angle split into three sections of thirty degrees each). You can eyeball it or you can use a protractor to mark your folding lines.
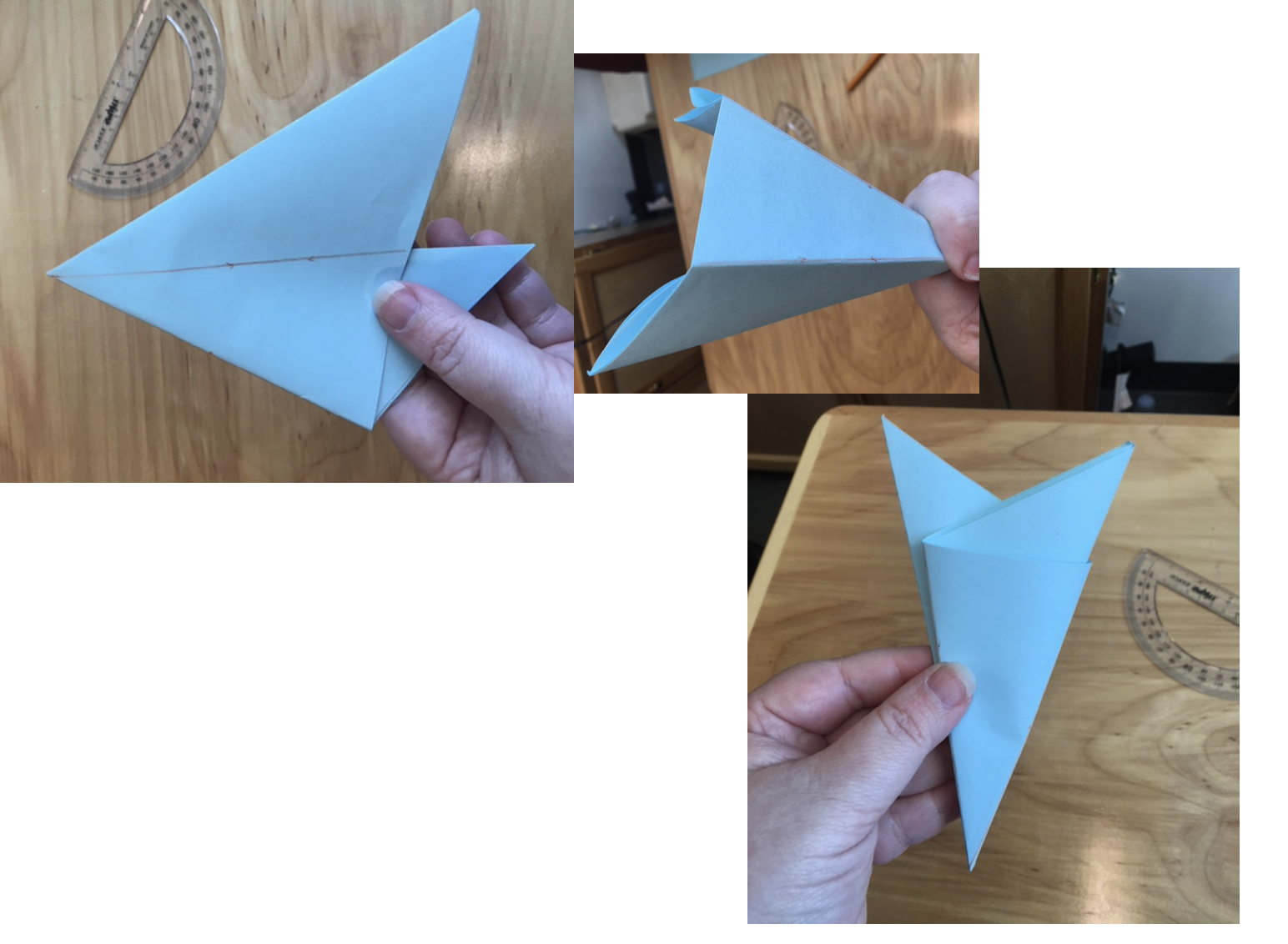
4. Cut off the resulting “bunny ears” and draw a desired pattern to cut through
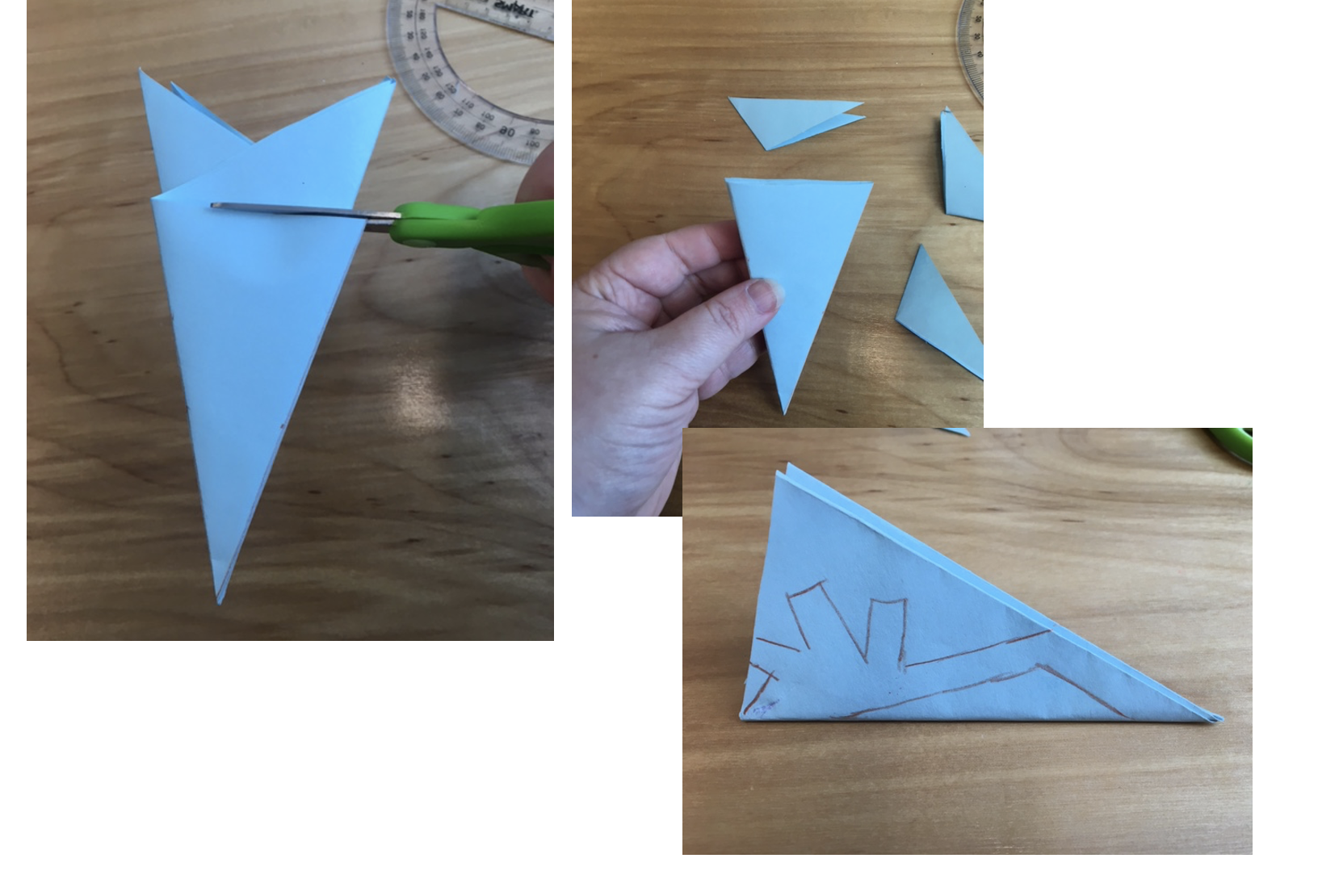
5. Cut through your drawn lines or eyeball the cuts. Be careful not to cut off the entire folded edge that keeps the structure together
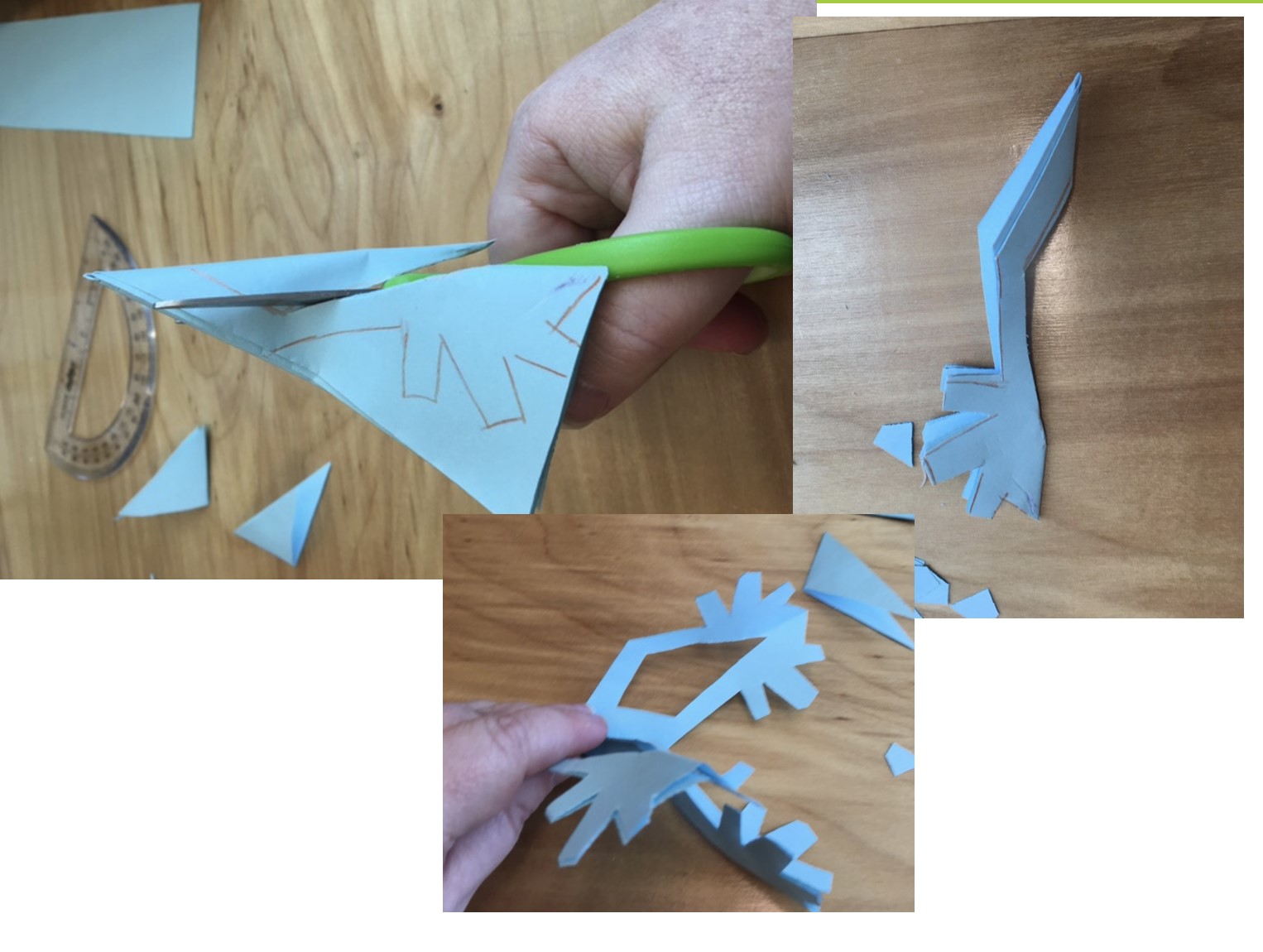
6. Unfold your snowflake creation. If it does not look right, you might have to cut out more negative space. You can place it under a heavy book or box to flatten it.

This link shows a good variety of snowflake patterns that you can make, but don’t be afraid to try your own creations.
I will “let it go” now... (shameless cultural reference most definitely intended). Regardless of your opinion about my snowflake vigilante ways, I hope you might appreciate learning a little more about the science behind these amazing structures that nature crafts for us, and I hope that you have some fun making “scientifically-accurately-sided” paper snowflakes. Happy Holidays!
